When
Bayer introduced aspirin in 1899, Cannabis was America’s number one painkiller. Until Cannabis prohibition began in 1937, the US
Pharmacopoeia listed Cannabis as the primary medicine for over 100
diseases. Cannabis was such an effective analgesic that the American
Medical Association (AMA) argued against prohibition on behalf of
medical progress. Since the herb is extremely potent and essentially
non-toxic, the AMA considered it a potential 'wonder drug'.
 In the 1800's, salicin from the bark of white willow was used to develop aspirin, acetylsalicylic acid. In combination with white willow's powerful anti-inflammatory plant compounds (flavonoids, like those found in Cannabis), salicin is thought to be responsible for the pain-relieving and anti-inflammatory effects of the herb. White willow appears to bring pain relief more slowly than aspirin, but its effects may last longer. The use of willow bark dates to the time of Hippocrates (400 BC) when people were advised to chew on the bark to reduce fever and inflammation. Willow bark has been used throughout the centuries in China and Europe and continues to be used today for the treatment of pain (particularly low back pain and osteoarthritis), headache and inflammatory conditions, such as bursitis and tendonitis.
In the 1800's, salicin from the bark of white willow was used to develop aspirin, acetylsalicylic acid. In combination with white willow's powerful anti-inflammatory plant compounds (flavonoids, like those found in Cannabis), salicin is thought to be responsible for the pain-relieving and anti-inflammatory effects of the herb. White willow appears to bring pain relief more slowly than aspirin, but its effects may last longer. The use of willow bark dates to the time of Hippocrates (400 BC) when people were advised to chew on the bark to reduce fever and inflammation. Willow bark has been used throughout the centuries in China and Europe and continues to be used today for the treatment of pain (particularly low back pain and osteoarthritis), headache and inflammatory conditions, such as bursitis and tendonitis.
 In
1980, 555 children died from Reye’s
syndrome.
Two years later the United States (US) Centres for Disease Control
(CDC)
advised “physicians
and parents of the possible increased risk of Reye syndrome
associated with the use of salicylates (aspirin) for children with
chickenpox and influenza-like illness”. That
same year, The American Academy of Paediatrics’ Committee on
Infectious Diseases issued a statement advising that “the
use of salicylates should be avoided for children suffering from
influenza or chickenpox” and
the Surgeon General issued a statement advising “against
the use of salicylates and salicylate-containing medications for
children with influenza and chickenpox".
In
1980, 555 children died from Reye’s
syndrome.
Two years later the United States (US) Centres for Disease Control
(CDC)
advised “physicians
and parents of the possible increased risk of Reye syndrome
associated with the use of salicylates (aspirin) for children with
chickenpox and influenza-like illness”. That
same year, The American Academy of Paediatrics’ Committee on
Infectious Diseases issued a statement advising that “the
use of salicylates should be avoided for children suffering from
influenza or chickenpox” and
the Surgeon General issued a statement advising “against
the use of salicylates and salicylate-containing medications for
children with influenza and chickenpox". In
2008, David Michaels published, Doubt
Is Their Product: How Industry’s Assault on Science Threatens Your
Health,
a controversial account of how US regulatory agencies were undermined
and public health endangered by unethical corporations and the
scientists who work for them. In his introduction, Michaels described
how aspirin manufacturers did everything they could to delay the
Food and Drug Administration (FDA)'s decision to place warning labels on aspirin bottles linking
the medicine to the child-killing disease, Reye’s syndrome. The
aspirin industry fought a warning label for five years during which
hundreds of children died. Michaels’ wrote, “Today,
less than a handful of Reye’s syndrome cases are reported each year
(less than two per year since 1994), no thanks to the corporations
which manufactured the product".
In
2008, David Michaels published, Doubt
Is Their Product: How Industry’s Assault on Science Threatens Your
Health,
a controversial account of how US regulatory agencies were undermined
and public health endangered by unethical corporations and the
scientists who work for them. In his introduction, Michaels described
how aspirin manufacturers did everything they could to delay the
Food and Drug Administration (FDA)'s decision to place warning labels on aspirin bottles linking
the medicine to the child-killing disease, Reye’s syndrome. The
aspirin industry fought a warning label for five years during which
hundreds of children died. Michaels’ wrote, “Today,
less than a handful of Reye’s syndrome cases are reported each year
(less than two per year since 1994), no thanks to the corporations
which manufactured the product".
Michaels
described this as “a public health triumph … but a
bitter-sweet one because an untold number of children died or were
disabled while the aspirin manufacturers delayed the FDA’s
regulation by arguing that the science establishing the link was
incomplete, uncertain and unclear. The industry raised seventeen
specific ‘flaws’ in the studies and insisted that more reliable
ones were needed. The medical community knew of the danger … but
parents were kept in the dark”. The drug makers worked with the
Reagan administration to delay “a public education program for
two years and mandatory labels for two more” while thousands of
children died.
Surgeon
General's Advisory on the Use of Salicylates and Reye Syndrome
Because
the use of salicylates such as aspirin for children with influenza
and chickenpox has been associated with Reye syndrome, the Surgeon
General advises against use of salicylate and salicylate-containing
medications for children with these diseases. The association of
salicylates with Reye syndrome is based upon evidence from
epidemiologic studies that are sufficiently strong to justify this
warning to parents and health care personnel.
First
recognised about 19 years ago, Reye syndrome is a rare, acute,
life-threatening condition characterised by vomiting and lethargy
that may progress to delirium and coma. Most commonly it occurs in
children who are recovering from viral infections, particularly
influenza and chickenpox. The CDC estimates that 600-1,200 cases occur each year in the United States,
most in persons between the ages of 5 and 16 years. Death occurs in
20%-30% of reported cases and permanent brain damage has also been
reported in survivors.
There
have been reports for several years suggesting an association between
Reye syndrome and the prior use of common medications. However, the
results of recent case-control studies have made it possible to
assess the association with specific drugs. These studies conducted
by state health departments suggest an association between prior
ingestion of aspirin and other salicylates and Reye syndrome ...
studies in Arizona and Michigan have been published … The
Surgeon General notes that the matter has been reviewed recently by
several groups from within and outside government.
- CDC, on the basis of its review of the available data and the recommendations of an advisory panel on February 12, 1982, stated that "until definitive information is available, CDC advises physicians and parents of the possible increased risk of Reye syndrome associated with the use of salicylates for children with chickenpox and influenza-like illness".
- The American Academy of Paediatrics' Committee on Infectious Diseases also has reviewed the data and in the June 1982 issue of Paediatrics issued a statement advising that the use of salicylates should be avoided for children suffering from influenza or chickenpox.
- A FDA working group audited the raw data in February 1982 from 3 studies conducted by state health departments and independently analysed the data. The FDA evaluation was discussed in an open public meeting sponsored by FDA, CDC and the National Institutes of Health on May 24, 1982. The meeting was attended by invited experts from the academic community, the drug industry and consumer organisations. It was the consensus of the scientific working group at the completion of the meeting that the new analysis supported the earlier evidence of an association between salicylates and Reye syndrome. As a result of this entire review process, the Surgeon General advises against the use of salicylates and salicylate-containing medications for children with influenza and chickenpox.*
*The
Surgeon General notes that the FDA will notify health professionals
through its Drug Bulletin, will develop lay-language information for
widespread distribution, and will take the steps necessary to
establish new labelling requirements for drugs containing
salicylates.
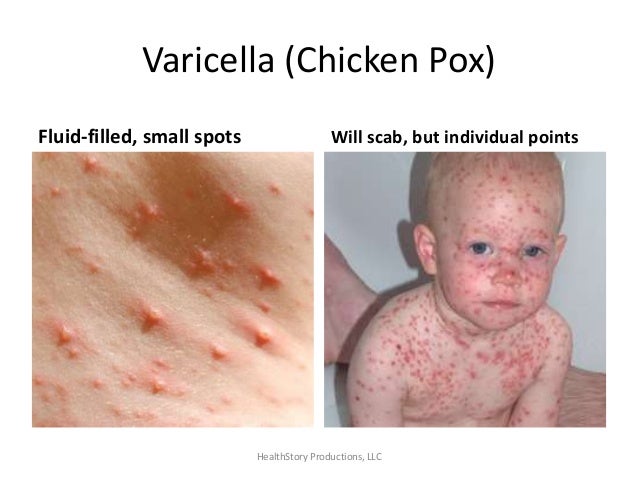 Reye's
syndrome is a rare but serious condition that causes swelling in the
liver and brain and most often affects children and teenagers
recovering from a viral infection, usually the flu or chickenpox.
Signs and symptoms such as confusion, seizures and loss of
consciousness require emergency treatment. Early diagnosis and
treatment can save a child's life. Aspirin has been linked with
Reye's syndrome, so use caution when giving aspirin to children or
teenagers. Though aspirin is approved for use in children older than
age 2, children and teenagers recovering from chickenpox or flu-like
symptoms should never take aspirin.
Reye's
syndrome is a rare but serious condition that causes swelling in the
liver and brain and most often affects children and teenagers
recovering from a viral infection, usually the flu or chickenpox.
Signs and symptoms such as confusion, seizures and loss of
consciousness require emergency treatment. Early diagnosis and
treatment can save a child's life. Aspirin has been linked with
Reye's syndrome, so use caution when giving aspirin to children or
teenagers. Though aspirin is approved for use in children older than
age 2, children and teenagers recovering from chickenpox or flu-like
symptoms should never take aspirin. Reye's
syndrome was first discovered in Australia in 1963 by pathologist R.
Douglas Reye and a few months later, rediscovered by G M Johnson in
the US. It wouldn't be truly recognised as a disease until 1973.
Reye's syndrome was nearly always fatal, involving non-inflammatory
encephalopathy and fatty degenerative liver failure, otherwise known
as brain and liver failure. While almost all the bodies organs are
affected, these two are the most common with Reye's Syndrome victims.
It is now extremely rare with only a single confirmed case in 2002
and only three suspected cases in 2009. It is not a contagious
disease.
Reye's
syndrome was first discovered in Australia in 1963 by pathologist R.
Douglas Reye and a few months later, rediscovered by G M Johnson in
the US. It wouldn't be truly recognised as a disease until 1973.
Reye's syndrome was nearly always fatal, involving non-inflammatory
encephalopathy and fatty degenerative liver failure, otherwise known
as brain and liver failure. While almost all the bodies organs are
affected, these two are the most common with Reye's Syndrome victims.
It is now extremely rare with only a single confirmed case in 2002
and only three suspected cases in 2009. It is not a contagious
disease.
The creation of aspirin gave birth to the modern pharmaceutical industry
and Americans switched from Cannabis in the name of 'progress'. But
it wasn't really progressive, it was regressive. Aspirin has a short
history, in comparison to Cannabis, as the drug of choice for the
self-treatment of migraines, arthritis and other chronic pain. It is
cheap, effective and legally available. But it is not as safe as
Cannabis!
History:
- Cannabis has been used for at least 5,000 years
- No one has ever overdosed on Cannabis (physiologically impossible)
- Aspirin has been used for over 100 years
- Approximately 500 people die every year across the US from taking aspirin
US
Law:
- Cannabis is a Schedule 1 drug, meaning the US government believes it is extremely dangerous, highly addictive and of no medical value
- Aspirin is available for 'pennies' and can be purchased by children at any drug, grocery or convenience store across America, and is often handed out free by people with no medical knowledge
Cannabis
Side Effects and Dangers:
- Persecution and prosecution due to the 'War on Drugs'
- Possible respiratory problems caused by the deposition of burnt plant material on the lungs (from smoking). This danger can be eliminated with alternate forms of consumption such as eating or vaporising Cannabis
- For two to four hours, Cannabis causes short-term memory loss, a slight reduction in reaction time and a possible reduction in cognitive ability in those new to partaking of the herb. These conditions DO NOT persist after the initial effects of the herb wears off
- Hunger
- Paranoia (caused mostly by erroneous illegality in various jurisdictions)
- Introspection
- Creative Impulse
- Euphoria
- Tiredness
- Forgetfulness
Aspirin
Side Effects and Dangers:
- When taken with alcohol, aspirin can cause stomach bleeding
- Reye Syndrome in children: fat begins to develop around the liver and other organs, eventually putting severe pressure on the brain. Death is common within a few days
- People with haemophilia can die
- People with hyperthyroidism suffer elevated T4 levels
- Stomach problems include dyspepsia, heartburn, upset stomach, stomach ulcers with gross bleeding, and internal bleeding leading to anaemia
- Dizziness, ringing in the ears, hearing loss, vertigo, vision disturbances and headaches
- Heavy sweating
- Irreversible liver damage
- Inflammation and gradual destruction of the kidneys
- Nausea and vomiting
- Abdominal pain
- Lethargy
- Hyperthermia
- Dyspepsia: a gnawing or burning stomach pain accompanied by bloating, heartburn, nausea, vomiting and burping
- Tachypnoea: Abnormally fast breathing
- Respiratory Alkalosis: a condition where the amount of carbon dioxide found in the blood drops to a level below normal range brought on by abnormally fast breathing
- Cerebral Oedema: Water accumulates on the brain. Symptoms include headaches, decreased level of consciousness, loss of eyesight, hallucinations, psychotic behaviour, memory loss, coma, and if left untreated, death
- Hallucinations, confusion and seizure
- Prolonged bleeding after operations or post-trauma for up to 10 days after last aspirin
- Aspirin can interact with some other drugs, such as diabetes medication, as aspirin changes the way the body handles these drugs and can lead to drug overdose and death
So
if safety is your concern, Cannabis is clearly a much better choice
than aspirin. If you eat it or vaporise it, Cannabis is the safest
painkiller the world has ever known.
 In 2014, 40 million Americans were advised that if they hadn't had a heart attack they should no longer take an aspirin a day! After many decades of promoting aspirin, the FDA said that if you'd not experienced a heart problem, you should not be taking a daily aspirin, even if you have a family history of heart disease. This represented a significant departure from the FDA's prior position on aspirin for the prevention of heart attacks. "The FDA has concluded that the data do not support the use of aspirin as a preventive medication by people who have not had a heart attack, stroke or cardiovascular problems, a use that is called 'primary prevention'. In such people, the benefit has not been established but risks - such as dangerous bleeding into the brain or stomach - are still present".
In 2014, 40 million Americans were advised that if they hadn't had a heart attack they should no longer take an aspirin a day! After many decades of promoting aspirin, the FDA said that if you'd not experienced a heart problem, you should not be taking a daily aspirin, even if you have a family history of heart disease. This represented a significant departure from the FDA's prior position on aspirin for the prevention of heart attacks. "The FDA has concluded that the data do not support the use of aspirin as a preventive medication by people who have not had a heart attack, stroke or cardiovascular problems, a use that is called 'primary prevention'. In such people, the benefit has not been established but risks - such as dangerous bleeding into the brain or stomach - are still present".
The FDA announcement was prompted by Bayer's request to change its aspirin label to indicate it could help prevent heart attacks in healthy individuals. Aspirin generated $1.27 billion in sales for Bayer in 2013 and from Bayer's request it appears they wanted everyone to be taking their drug. But the FDA said, 'not so fast', and rightly so. Evidence in support of using aspirin preventatively went from weak to weaker to nonexistent. Even 'low-dose aspirin' (LDA) may do far more harm than good.
Withdrawal:
Presence and severity of characteristic withdrawal symptoms.
Reinforcement:
A measure of the substance’s ability, in human and animal tests, to
get users to take it again and again and in preference to other
substances.
Tolerance:
How much of the substance is needed to satisfy increasing cravings
for it and the level of stable need that is eventually reached.
Dependence:
How difficult it is for the user to quit, the relapse rate, the
percentage of people who eventually become dependent, the rating
users give their own need for the substance and the degree to which
the substance will be used in the face of evidence that it causes
harm.
Intoxication:
Though not usually counted as a measure of addiction in itself, the
level of intoxication is associated with addiction and increases the
personal and social damage a substance may do.
Adapted
from;
Marijuana
Safer Than Aspirin, Reyes
Syndrome, Surgeon General's Advisory on the Use of Salicylates and Reye Syndrome, FDA Reverses Its Position on Daily Aspirin, 25
Years of Global Warming Denial(2)


No comments:
Post a Comment